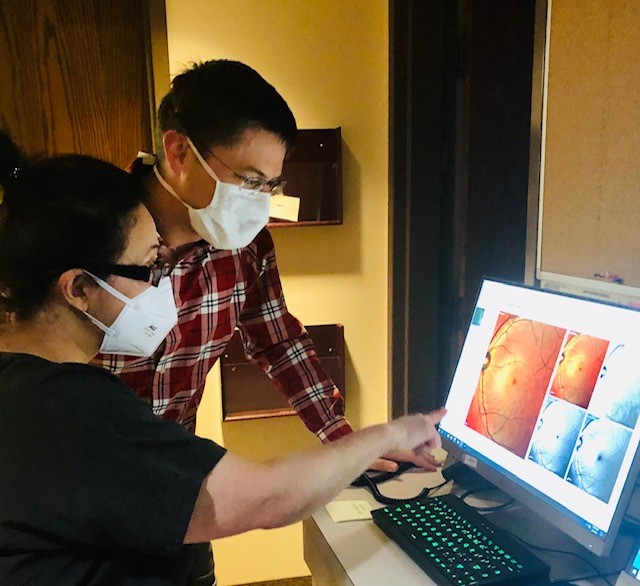
Patients benefit from the Eye Institute’s involvement in numerous Food and Drug Administration (FDA) national clinical research trials. Providing access to these national studies keeps the community updated on the latest medical and surgical advances in eye care. The FDA regulates the clinical trials offered at the Eye Institute to protect our patient’s rights, safety, and welfare. We offer clinical trials that explore the latest treatments for macular degeneration, diabetic retinopathy, macular edema, uveitis, dry eye, high eye pressure, and many others. The physicians at Black Hills Regional Eye Institute in Rapid City, South Dakota have been participating in clinical trials for several years.